According to official website data of the State Food and Drug Administration, as of January 21st, 2016, due to the untrue and incomplete clinical trial data, the number of drug registration applications rejected by the State Food and Drug Administration and withdrawn by pharmaceutical companies for self-examination was as high as 1,184, accounting for 73% of the total number required for self-examination. If 165 clinical exemptions are deducted, the proportion will reach 81%.
The staff of the food and drug department said that during the verification, it was found that the clinical trial data of many drugs were incomplete, the analysis data had no differential trajectory, and some data could not be traced back. Other companies deliberately concealed or omitted the adverse reaction records, and revised the test data that failed to meet expectations.
At the beginning of September, a report that "80% of the clinical data of new drugs are involved in fraud, and the regulatory links are falling behind" caused a shock. The direct consequence of clinical data fraud is poor efficacy. To some extent, clinical data fraud affects everyone.
According to the news, over 80% of the clinical data of new drugs have been found to be fake since china food and drug administration started the self-inspection and verification of drug clinical trial data one year ago, and the regulatory links behind them have fallen behind, and the violations of relevant subjects such as pharmaceutical companies, intermediaries and doctors are prominent.
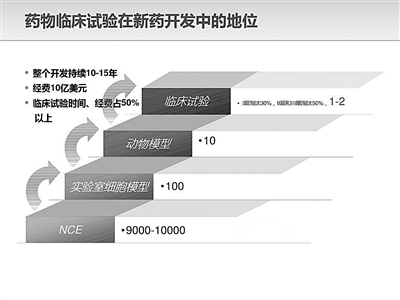
The data of drug clinical trials are of great significance in drug research and development innovation, and standardized clinical trials with reliable data are the key link to ensure drug quality. As the last link of new drug research and development, whether the drugs developed with tens of millions or even hundreds of millions of yuan have curative effect and safety depends mainly on the experimental data. The falsification of drug clinical trial data will not only bring huge security risks to patients’ medication, but also affect the innovation and upgrading of China’s pharmaceutical industry.
status
Fraud in clinical trials is no longer news.
The results of clinical trials are not only the only standard to test the safety and effectiveness of drugs, but also the key basis for drug registration and listing. Pei yu, director of the Department of Pharmacy, School of Pharmacy, Jinan University, said that clinical trials with strict specifications and reliable data are a key safety barrier before the drug goes on the market, and also the core basis for drug supervision departments to support the drug listing. However, the survey found that it is no longer news that Chinese drugs were faked in the clinical trial stage before listing.
Data fraud began in preclinical research. "Before a drug goes on the market, human clinical trials must be done. However, from the moment the subjects were selected to join the group, fraud occurred all the time. " The person in charge of a pharmaceutical company, who asked not to be named, said that clinical trials often adopt the method of "competitive enrollment". The number of participants in the group will directly determine the doctor’s signature ranking and the amount of scientific research funds in the future. If a project needs 100 patients who meet the test requirements, but it does not get enough people before the planned date, the test may start with only 80 or even 60 people. In the face of lack, data can only be fabricated from scratch.
Not only that, the actual operation record is not true and it is also a common means of fraud. According to a survey conducted by China Journal of Clinical Pharmacology in 2013 on domestic experimental institutions, the proportion of non-standard experimental records is as high as 85.7%, which is the most problematic link in clinical trials, and only 1/7 institutions in China have not found any problems.
Fraud may appear in any link of clinical trials.
It is a consensus in the medical field that generic drugs should be equal to the original drugs in dosage form, specification, quality, efficacy and indications. Some pharmaceutical companies launch generic drugs in order to maximize profits, but the necessary link of clinical trials is often "ignored". The boss of a large biopharmaceutical company revealed that 90% of domestic generic drugs could not meet the original research standards. In order to pass the evaluation, it is very common to conceal data and selectively use data, and pharmaceutical companies even modify data in conjunction with doctors.
"The clinical data of drugs are untrue and irregular, and the reasons may not all be caused by enterprises." The general manager of a pharmaceutical company in Zhejiang said that there are many reasons and motivations, which may appear in any link of the whole chain of clinical trials. On the one hand, China’s current laws and regulations on drug clinical trial supervision are still not perfect; On the other hand, the number of drug clinical trial institutions is too small, which is seriously mismatched with the number of projects that need to carry out clinical trials every year, which leads to the overwhelming burden of clinical trial institutions and affects the quality and standardization of clinical trials.
question
Data fraud supervision is still "soft"
The extremely important data in clinical trials of new drugs are often ignored nowadays. In foreign countries, data statistics need to spend hundreds of thousands or even millions of RMB, while data statistics in China hospitals only need to spend tens of thousands of RMB.
China implements the qualification certification system for clinical trial institutions, that is, only clinical institutions that have passed the examination and approval of drug supervision departments can participate in clinical trials. The actual situation is often that it is very difficult for new drug trials to enter phase I clinical trials, but once they enter, they will be smooth all the way.
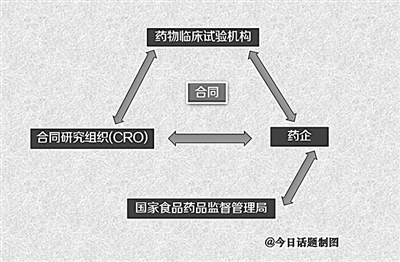
Traditionally, pharmaceutical companies often send inspectors (CRA) to supervise clinical trial institutions. After the clinical trial, CRA will collect the approved data and hand it over to the data processing institution for analysis and collation, and finally form the data and report submitted to the Drug Evaluation Center of the Food and Drug Administration for review. However, due to the lack of third-party supervision and checks and balances, hospitals as clinical trial institutions have frequent problems. In order to achieve the purpose of approval, some pharmaceutical companies will also modify the data in conjunction with statistical units. Although the General Administration clearly stipulates that if the clinical research data is fraudulent, the applicant’s new application for drug registration will not be accepted within 3 years, and the clinical trial data of the person directly responsible for participating in the research or organizing the research will not be accepted within 10 years. However, for a series of data fraud problems, supervision is still "soft".
Dr Liao Guojuan, CEO of Suzhou Jinweizhi Biotechnology Co., Ltd. said that in the United States, once data fraud occurs, pharmaceutical companies and R&D personnel will be blacklisted and will not be allowed to enter related fields for life.
counter-measure
New drug research and development must take the right path
Since April 2015, a number of new policies for drug trial reform have been introduced almost every month. On November 11th, last year, china food and drug administration also published the Announcement of china food and drug administration on Several Policies of Drug Registration Review and Approval in its official website, and put forward ten heavy new regulations, such as improving the approval standard of generic drugs and optimizing the review and approval of clinical trial applications. This may be a blessing for the creation of real new drugs with clinical value. However, after a lapse of one year, the news that 80% of the clinical data of new drugs are suspected of being falsified still broke out, which has to make people ponder: which link has gone wrong?
For the current situation of new drug research and development, the general manager of a pharmaceutical company in Nanjing feels very helpless: formal enterprises take the right path, only to find that enterprises that resort to deceit and cut corners are faster than their own approval.
The president of a biotechnology company in Shenzhen also said that many innovative companies that focus on drug research and development have made an innovative drug for ten years, and the data have been carefully made, but they have suffered losses when competing with companies that falsify data. "Strict review of clinical research data should not only be implemented early, but also be promoted. Although the progress of regular products may be affected in the short term, after the normalization, on the whole, it is conducive to the healthy development of innovative enterprises. "
Encourage real innovation with the strictest system
Some people in the medical field believe that there are two negative arguments to be vigilant at present: first, the introduction of policies should conform to China’s national conditions, and the requirements for innovation at this stage should not be too advanced; Second, the policy cannot be uniform, and some clinical data are just not standardized, not fraudulent, and should be allowed to exist.
In this regard, experts suggest that to ensure the authenticity of drug clinical data, we can refer to the advanced and mature practices in Europe and America, and have zero tolerance for clinical data fraud and other chaos, so as to effectively ensure the safety of patients’ medication; At the same time, more doctors should be allowed to engage in drug clinical trials and control the quality of clinical trials; Establish a third-party supervision system and strengthen supervision. In addition, electronic information technology can also be used to establish a database of drug clinical trials, requiring clinical institutions to report clinical trial data in real time and make it public, so as to realize universal supervision.
Authorities in the industry said that at present, it is a critical period to control "bad money drives out good money" in the field of drugs, and real innovation must be encouraged with the strictest system. Therefore, it is called for the revision of the "Quality Management Standards for Drug Clinical Trials" and other laws and regulations as soon as possible to further improve the system supervision; At the same time, the internal supervision system of pharmaceutical companies should be established to truly form core competitiveness through innovation. (Reporter Li Ying)